CIQTEK SEM Supports Publication in Advanced Functional Materials on Temperature-Controlled Adhesive Hydrogels
Professor Lai Yuekun’s team from Fuzhou University has conducted innovative research addressing the urgent demand for strong adhesive hydrogels in fields such as wearable sensors, soft robotics, tissue engineering, and wound dressings. Currently, interface adhesive materials face two major technical challenges: firstly, difficulty in achieving rapid and reversible switching between adhesive and non-adhesive states; secondly, poor adhesion performance in multi-liquid environments. Recently, the team conducted in-depth studies using the CIQTEK scanning electron microscope.
The PANC/T hydrogel was synthesized from acrylamide (AAm), N-isopropylacrylamide (NIPAM), a micellar solution composed of sodium dodecyl sulfate/methyl octadecyl methacrylate/sodium chloride (SDS/OMA/NaCl), and phosphotungstic acid (PTA). Dynamic interactions between PNIPAM chains and SDS enabled on-demand adhesion and separation. Further soaking in Fe³⁺ solution produced the PANC/T-Fe hydrogel, which achieves strong adhesion in various wet environments. This resulted in the development of an intelligent interface adhesive hydrogel with rapid responsiveness, capable of controlled adhesion and separation under different humidity conditions.
The research was published in Advanced Functional Materials under the title "Temperature-Mediated Controllable Adhesive Hydrogels with Remarkable Wet Adhesion Properties Based on Dynamic Interchain Interactions."
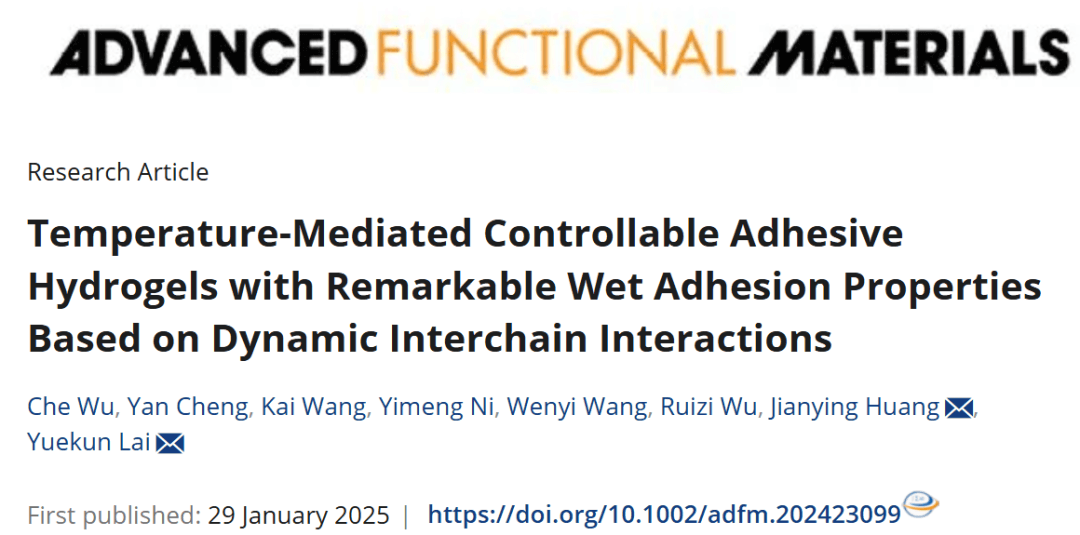
Synthesis and Structural Characteristics of Controllable Adhesive Hydrogel
PANC/T-Fe hydrogel is synthesized by copolymerization of hydrophilic AAm, amphiphilic NIPAM, and hydrophobic OMA. PTA acts as a crosslinker, forming hydrogen bonds with amino groups on polymer chains to establish a stable network. The team discovered that interactions between NIPAM and SDS are critical to the hydrogel’s temperature-sensitive adhesion. At lower temperatures, SDS crystallizes and adheres to PNIPAM chains, hindering adhesive functional groups from interacting with substrates and reducing adhesion. As temperature rises, SDS crystals melt, improving contact between adhesive groups and substrates and significantly increasing adhesion. PTA enhances adhesion at higher temperatures by physically interacting with polymer amino groups; this interaction weakens upon heating, softening the hydrogel and generating more adhesive sites. The dynamic regulation between polymer chains enables reversible, on-demand adhesion.
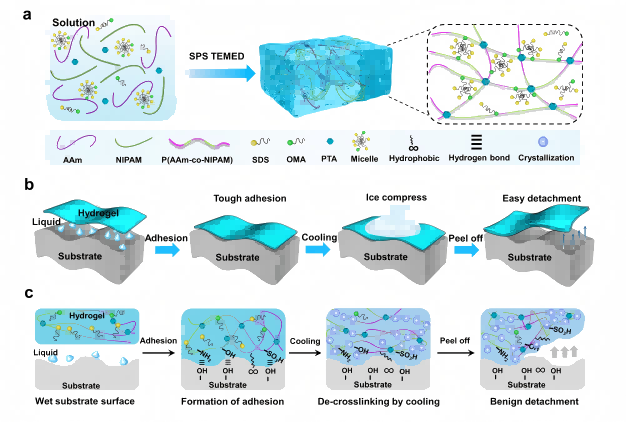 Figure 1. Hydrogel synthesis and mechanism of reversible wet adhesion.
Figure 1. Hydrogel synthesis and mechanism of reversible wet adhesion.
Temperature Regulation Mechanism of Adhesion Performance
Through comparative experiments, the team confirmed that the synergistic effect of NIPAM and the micellar solution is key to the hydrogel’s temperature-sensitive adhesion. Differential Scanning Calorimetry (DSC) results indicate the temperature response is unrelated to NIPAM’s Lower Critical Solution Temperature (LCST), but influenced by NIPAM-SDS interactions, which alter SDS crystallization temperature. In situ FT-IR testing revealed that increasing temperature weakens interchain hydrogen bonds, releasing more adhesive groups and enhancing adhesion. Rheological analysis further verified temperature-dependent changes in molecular interactions, causing the hydrogel to shift from rigid to flexible.
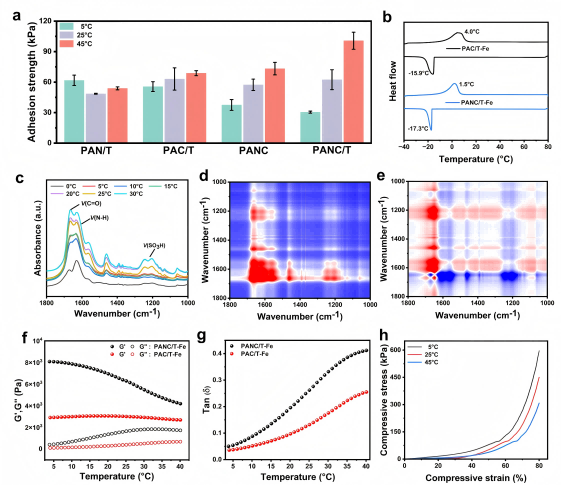 Figure 2. Mechanism study of temperature-sensitive adhesion.
Figure 2. Mechanism study of temperature-sensitive adhesion.
On-Demand Adhesion and Strong Wet Adhesion Performance
PANC/T-Fe hydrogel exhibits on-demand adhesion without external energy input, achievable by simple ice application. At room temperature (25°C), the hydrogel is soft and highly adhesive, making it difficult to peel from glass without leaving residue. Ice treatment enhances internal cohesion and elasticity, facilitating benign detachment and reducing adhesion strength. Adhesion remained stable over multiple cycles between 5°C and 25°C, demonstrating good reversibility. The hydrogel’s controllable adhesion under various environments holds significant potential in tissue healing, material repair, and wet-environment actuators.
Figure 3. Performance testing of reversible adhesion.
Wet Adhesion Performance in Various Liquid Environments
The hydrogel also performs excellently in liquid environments. The copolymer chains contain both hydrophilic and hydrophobic units; after Fe³⁺ treatment, these segments migrate and rearrange on the surface, enabling strong adhesion in both water and oil. Using CIQTEK SEM3100, the team observed structural changes before and after Fe³⁺ soaking, confirming polymer network rearrangement. Studies on NIPAM and PTAs’ influence showed their combined effect yielded outstanding adhesion in dry, aqueous, and oily environments, with adhesion strengths reaching 121 kPa, 227 kPa, and 213 kPa, respectively. The hydrogel strongly adheres to various substrates, including glass, metal, and wood, and maintains good adhesion in multiple organic solvents and aqueous solutions.
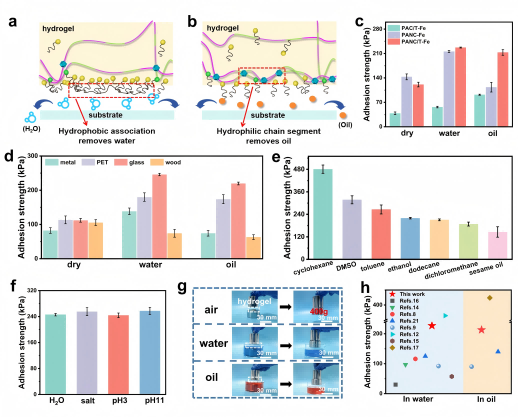 Figure 4. Wet adhesion performance in various liquid environments.
Figure 4. Wet adhesion performance in various liquid environments.
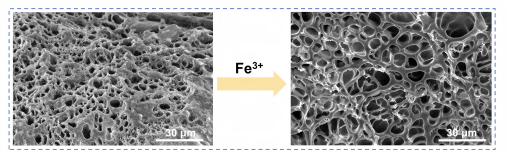 Figure S10. SEM images of hydrogel cross-section before and after Fe³⁺ treatment showing network loosening.
Figure S10. SEM images of hydrogel cross-section before and after Fe³⁺ treatment showing network loosening.
Repair Performance on Damaged Materials
PANC/T-Fe hydrogel has broad application prospects for the temporary repair of damaged materials. For example, in boat model leak repair tests, the hydrogel quickly stops liquid leakage; the repaired boats withstand certain weights without leakage. When repairing damaged substrates in water and oil, the hydrogel endures maximum burst pressures of 57 kPa and 49 kPa, respectively. Ice application allows easy removal without residue, a valuable feature for biomedical and smart material applications, demonstrating great practical potential.
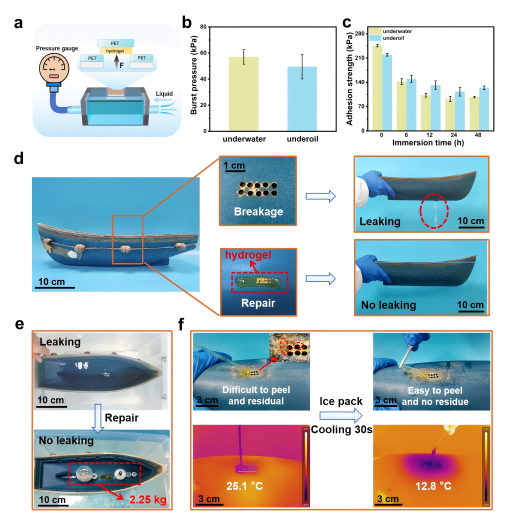 Figure 5. Temporary repair performance of PANC/T-Fe hydrogel.
Figure 5. Temporary repair performance of PANC/T-Fe hydrogel.
This study successfully synthesized PANC/T-Fe hydrogel featuring strong adhesion in various environments and reversible on-demand adhesion. It elucidated how dynamic interchain interactions influence adhesion performance, providing theoretical guidance for novel intelligent adhesive materials. The on-demand adhesion requires no external energy, achievable by ice application, offering a new approach for intelligent adhesives in liquid environments. This innovative control of adhesion performance is expected to enable broad applications and advance smart adhesive technologies, offering new solutions to adhesion-related challenges.
